News Story
Technology to deliver images of birth defect as it happens
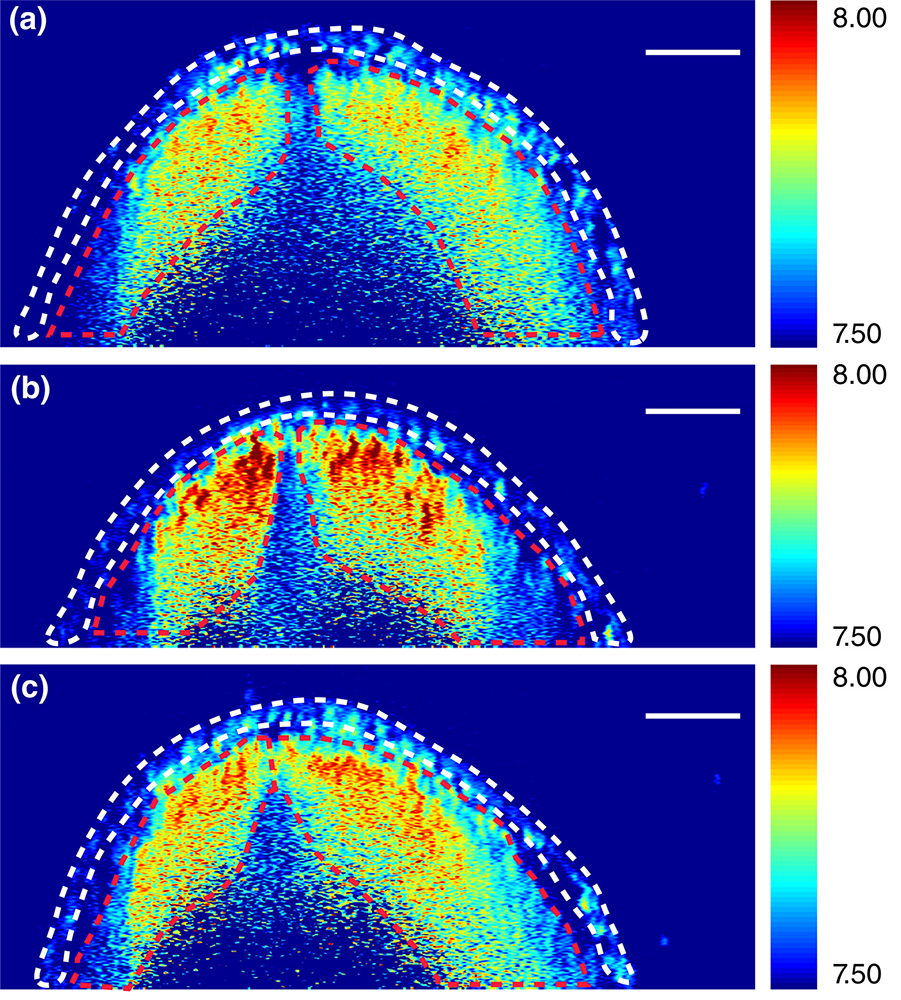
Brillouin images of neural tube cross sections at different levels within an embryo. DOI: 10.1002/bdr2.1389
In the first few weeks of pregnancy and often before a woman even realizes she’s pregnant, an embryo will have already developed a neural tube, a hollow structure made of cells that will eventually become the brain and spinal cord. Now, with $3.2 million from the National Institutes of Health, Giuliano Scarcelli—an assistant professor in the A. James Clark School of Engineering’s Fischell Department of Bioengineering—is part of an effort to tackle the evolutionary anomaly of why the neural tube closes in most embryos but remains open in others, leading to birth defects such as spina bifida and anencephaly.
Neural tube defects are the second most common structural birth defect in humans, affecting upwards of 500,000 pregnancies worldwide and approximately 2,400 pregnancies each year in the United States.
Embryonic development involves the interplay of driving forces that shape the tissue and the mechanical resistance that the tissue offers in response. However, tissue biomechanics is not well understood because of the lack of techniques that can quantify the stiffness of tissue in high resolution and without manipulating or damaging the tissue.
Led by Professor Kirill Larin of the University of Houston and together with Professor Richard Finnell of the Baylor College of Medicine, the research team including Scarcelli will create new technology combining Brillouin spectroscopy and optical coherence tomography (OCT) to deliver 3D images of the mechanical factors at play when the neural tube closes and—in so many cases—when it does not.
Most commonly used to examine the retina, OCT is an imaging technology that uses light waves to take cross-section pictures. Brillouin spectroscopy is a light scattering technology that will sense tissue mechanical properties, which growing evidence has suggested is critical to the neural tube’s success in closing. Scarcelli has invented and led the development of Brillouin microscopy over the past 10 years.
“Generally, to characterize the stiffness of a material, you need to stretch and pull that material—which usually destroys it. This new Brillouin technology enables us to determine the mechanical properties of embryonic tissue in a noninvasive way and as that tissue grows,” says Scarcelli.
The work fills a significant data gap in understanding neural tube closure biomechanics. The research team says it could have a dramatically positive impact on our understanding of neural tube defects and drive novel treatments for at-risk embryos.
“It’s still one of the great mysteries of life—no one on Earth knows how this happens, and that is really exciting to us,” says Larin, “because we will be the ones to find out.”
Published September 27, 2018









