News Story
Findings Published on Ion Adsorption Energetics
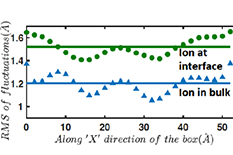
Research results demonstrating that the RMS (Root Mean Square) of the fluctuations of the Capillary Waves with the ion at the air-water interface versus the ion at the bulk. Credit: Y. Wang et al., "Ion at air-water interface enhances capillary wave fluctuations: Energetics of ion adsorption", Journal of the American Chemical Society, DOI: 10.1021/jacs.8b06205, 2018.
A team of graduate students led by Mechanical Engineering Assistant Professor Siddhartha Das has published findings pinpointing the principles dictating ion adsorption at the air-water interface in the Journal of the American Chemical Society. The lead author of the paper is Professor Das’ Ph.D. student Mr. Yanbin Wang. Other students from Prof. Das’ group involved in this work are Mr. Shayandev Sinha (currently a postdoctoral researcher at Harvard University), Mr. Parth Rakesh Desai, and Mr. Haoyuan Jing.
Understanding the ion adsorption at the air-water interface is central to a large number of processes such as regulating the size of the atmospheric aerosols that dictate thunderstorm activity, production of lightning and precipitation, hydrolysis reaction that leads to acid rain, ozone formation in the polluted marine boundary layers, and dictating the preferential adsorption of biological molecules (e.g., proteins) at the air-water interface. Researchers have produced a huge volume of work over the years that has tried to unravel the mechanisms of the adsorption of different ions at the air-water interface.
The present paper challenges some of the existing ideas concerning the mechanisms of ion adsorption at the air-water interface. Das and his students demonstrate that contrary to classical notion, an ion approaching the air-water interface increases the fluctuations of the capillary waves (CWs) associated with the air-water interface, which in turn dictate the overall change in the Gibbs free energy (dictated by the corresponding changes of the enthalpy and entropy). For example, dictated by the wave structure of the CW fluctuations, there is a large decrease in the pressure-volume (PV) work associated with the ion adsorption at the air-water interface.
This decrease in the PV work is responsible for the decrease in the enthalpy associated with the ion adsorption process, unlike the process of the re-distribution of the water molecules from the interface to the bulk as hypothesized by the existing studies. Moreover, the entropy lowering associated with the ion adsorption processes is associated with the lowering of the mixing entropy, caused by the structure of the CWs. This too contradicts the standard idea that the decrease in the entropy is caused by the reduction of the CW fluctuations. These distinctly new inferences of the present study arise from the consideration of an appropriately large simulation box volume that the existing studies have not considered and have accordingly enforced an artificial suppression of the CWs.
"Most of the existing studies of ion adsorption at the air-water interface have been riddled with issues of an inappropriate capture of the interactions between the ion and the CWs,” points out Das. “Our paper will pave the way for probing the interactions between ion and air-water interface in a novel manner with implications to better understand the several processes dictated by the ion adsorption at the air-water interface.”
Published September 25, 2018









